01-04-2021 By Quick Biology
In 2020, The Royal Swedish Academy of Sciences awarded the Nobel Prize in Chemistry 2020 to Emmanuelle Charpentier and Jennifer A. Doudna, who have discovered CRISPR/Cas9 system, a genetic scissor for rewriting the code of life. David Liu’s lab at the Broad Institute of MIT & Harvard has been inventing new CRISPR gene-editing systems without double-strand DNA break in CRISPR/Cas9 system. In 2016, they invented “base editing”, which seamlessly does four transition editing (C to T, G to A, A to G, and T to C). In 2019, they gave the world “prime editing”, which is devised to achieve all 12 base-to-based conversions, in addition, it can mediate targeted insertions and deletions.
Prime editing is a much more promising new technology to correct most genetic defects. The scientific community has quickly tested and used prime editing in human cell lines, plant cells, and mouse embryonic cells when David Liu’s lab shared all materials, deposited all relevant constructs in Addgene. Adult stem cell-derived organoids exhibit important functional properties of organs, allowing modeling of monogenic disease. In Nature Communications, researchers from Dutch firstly applied primer editing in human patient-derived organoids. First, they generate precise in-frame deletions in the gene encoding beta-catenin (CTNNB1) that result in proliferation independent of Wnt-stimuli, mimicking a mechanism of the development of liver cancer (Fig.1). Second, they did Biallelic DGAT1 mutations using prime editing systems in patient-derived intestinal organoids, they found the correcting disease-causing indel mutations can restore the function of genetic defects (Fig.2). By performing whole genome sequencing of prime-edited clonal organoids, they revealed the absence of genome-wide off-target effects underscoring the therapeutic potential of the versatile and precise gene-editing strategy (Fig.3).
Figure 1: Prime editing efficiently creates deletions and point mutations in organoids. (ref1)
a Schematic overview of the workflow and timeline to generate precise mutations in organoid cells using prime editing (PE3). b pegRNA design, Sanger validation in a clonal organoid with monoallelic edit, and editing efficiency of a 5-bp deletion in HEK3 in liver and intestinal organoids. c pegRNA design, Sanger validation of monoallelic edit, and editing efficiency for a C→G substitution in HEK3 in liver organoids. d pegRNA designs for the generation of in-frame deletions in the β-TrCP region of CTNNB1. e Brightfield images of liver organoid cells transfected with plasmids from d after Rspo1 withdrawal for 2 weeks. f Sanger validation of precise 6-bp deletions in all picked clones from CTNNB1 pegRNA S1E1 that continue growing in -Rspo1 conditions. g Quantification of organoid outgrowth in e. p < 0.0001 in a one-way ANOVA with Holm–Sidak correction. h Comparison of editing efficiencies and generation of unwanted byproducts in different cell types by high-throughput sequencing (HTS). FA fatty acids, PBS primer binding site, RTT reverse transcriptase template, NC negative control.
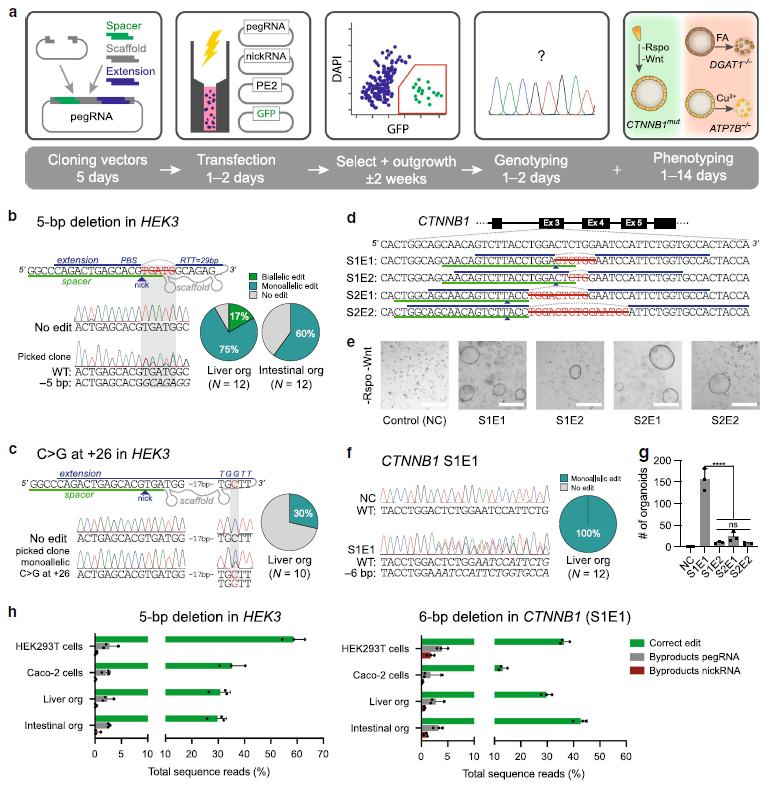
Figure 2: Prime editing functionally corrects disease-causing indel mutations in intestinal and liver organoids. (Ref1)
a Schematic overview of the DGAT1 disease mechanism. b Sanger validation of biallelic DGAT1S210del mutations in patient-derived intestinal organoids, pegRNA design, and Sanger validation of successful biallelic correction by PE3. c Brightfield images of healthy control- and DGAT1S210del patient-derived intestinal organoids (±PE3) after exposure to 4mM OA for 24 h and subsequent passaging (split). Quantification of corrected alleles in patient organoids after PE3 and OA selection; d Quantification of DGAT1S210del patient organoid survival upon exposure to 4mM OA, after targeting with different PE3 or PE3b plasmids. e Western blot of DGAT1 in DGAT1S210del, healthy control, and PE3-corrected DGAT1S210del organoids. f Quantification of correct edits and unwanted indels by PE3 and Cas9-initiated HDR in DGAT1S210del organoids. g Comparison of PE3 and adenine base editing (ABEmax-NG) to correct the ABCB11R1153H and SERPINA1E342K mutations in liver organoids from patients with BSEP-deficiency and alpha-1-antitrypsin deficiency, respectively. h Schematic overview of the Wilson disease (ATP7B deficiency) mechanism. i Sanger validation of biallelic ATP7BS430fs mutations in patient-derived liver organoids, pegRNA#2 design to target this mutation, and Sanger validation of successful monoallelic correction by PE3. j Cell death in ATP7BWT and ATP7BKO liver organoids after incubation with Cu2+ for 3 days. k Brightfield images of ATP7BS430fs-patient organoid survival upon exposure to 0.25mM Cu2+ for 3 days, after transfection with different PE3 plasmids. “at −39” stands for nicking sgRNA at position −39. ER endoplasmic reticulum, FFA free fatty acid, Ex exon, NC negative control, OA oleic acid, PE3+ m PE3 with the introduction of PAM mutation, HDR homology-directed repair, ABE adenine base editor, PI propidium iodide.
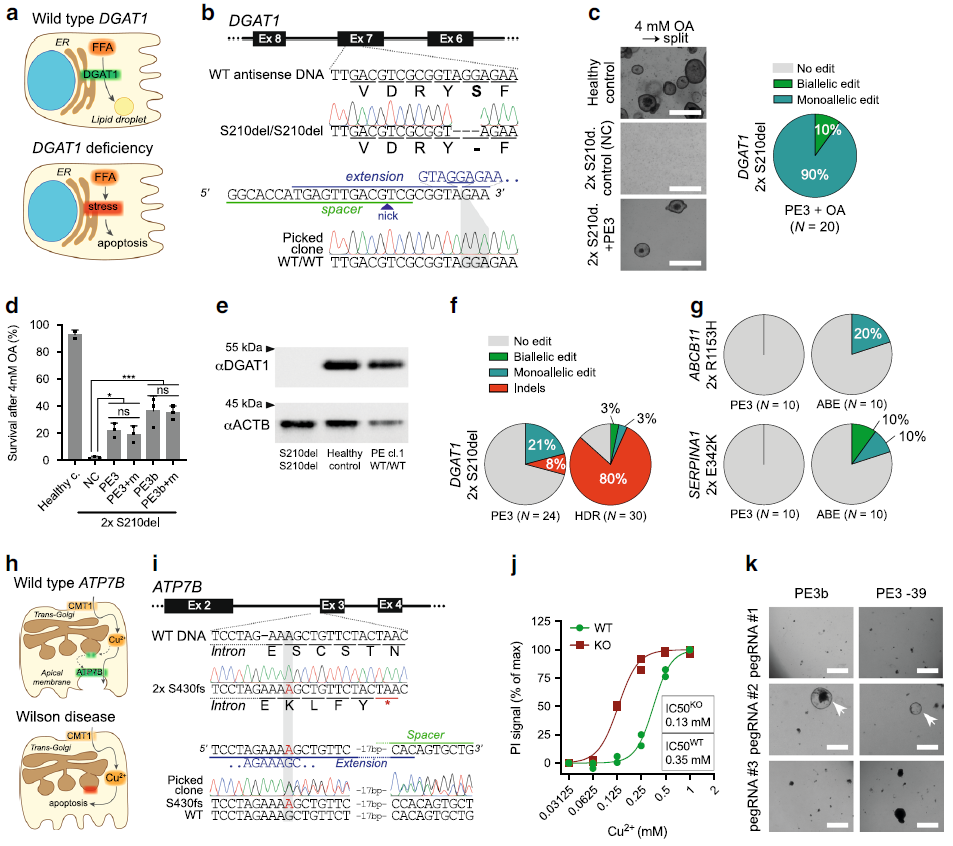
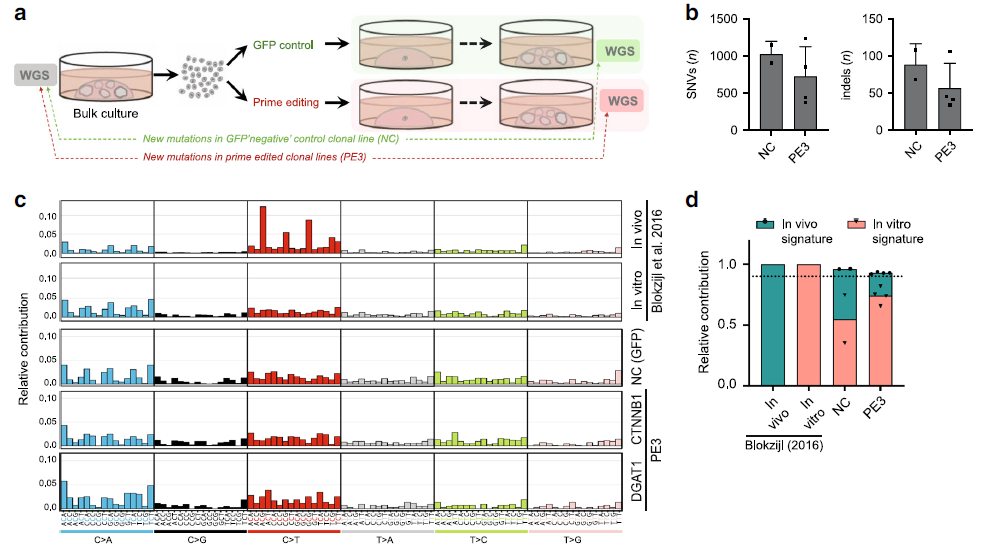
Figure 3: Prime editing induces no genome-wide off-target effects. (Ref1)
a Schematic overview of the protocol used to identify mutations induced by prime editing (PE3). WGS was performed for one unedited negative control and two prime-edited clonal lines for both DGAT1 (intestinal organoids) and CTNNB1 (liver organoids). b Total number of single-nucleotide variants (SNVs) and insertions and deletions (indels) in control (NC) and prime-edited (PE3) clonal organoid lines (n = 2 and n = 4 biologically independent samples, respectively). Error bars represent S.D. c Mutational signature analysis by relative contribution of context-dependent mutation types in an in vivo (n =6) and in vitro (n = 6) data set (Blokzijl et al.16) and in control and prime-edited clonal organoid lines. d Relative contribution of known in vivo and in vitro mutational signatures from Blokzijl et al.16 in control (NC) and prime-edited (PE3) clonal organoid lines (n= 2 and n = 4 biologically independent samples, respectively). Dotted line at 0.9 indicates highly accurate signature reconstruction. NC negative control, WGS whole-genome sequencing.
Quick Biology can assist you with CRISPR genome editing screening. Find More at Quick Biology.
See resource:
1. Schene, I. F. et al. Prime editing for functional repair in patient-derived disease models. Nat. Commun. 11, 1–8 (2020).